Ethylene Oxide Sterilization Residuals
Overview of Ethylene Oxide EO or EtO Residuals Sterilizing medical devices with ethylene oxide EO is a common practice primarily due to its extensive material compatibility. Ethylene oxide also known as EO or EtO is a low temperature gaseous process widely used to sterilize a variety of healthcare products such as single-use medical devices.

Ethylene Oxide Sterilization Advamed
Ethylene oxide sterilization residuals 1 Scope This part of ISO 10993 specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices procedures for the measurement of EO and ECH and methods for determining compliance so that devices may be released.
Ethylene oxide sterilization residuals. ISO 10993-72008 specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices procedures for the measurement of EO and ECH and methods for determining compliance so that devices may be released. Ethylene oxide is a dominant agent in the sterilization of medical devices due to its effectiveness and compatibility with most materials. Tests for the presence of EO and Ethylene Chlorohydrin ECH residuals according to ANSIAAMIISO 10993-7 Biological evaluation medical devices Part 7.
Finally the product goes into a heated aeration chamber where it dwells at approximately 110 to 115 F with air circulation to further reduce the residual EO absorbed into the product. In general sterilization validation is required to demonstrate that the devices are free of viable microorganisms. Iso 10993-7 ethylene oxide sterilization is used by stagfarmporcent in Iso 10993-7 Ethylene Oxide Sterilization Residuals Pdf LINK.
Such offgassing phenomena are of particular concern since they extend the population that is routinely exposed beyond the. This test helps manufacturers demonstrate the safety of products sterilized by EO by determining compliance with accepted residual limits. The advantages and disadvantages as well as its.
However the aeration time that optimizes the removal of the remaining EO when a rigid steriliz. Specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices and procedures for the measurement of EO and ECH. All EO Validations require residual testing.
Of Ethylene Oxide Union Carbide Chemicals Patent for sterilization of spices Lloyd Hall Use in sterilization of materials Dr. Ethylene oxide EO is used to sterilize Oxygenator and Tubing applied to heart surgery. First EO residue Extraction of medical device in DI H2O after it comes out of sterilization chamber At different interval of times.
These ETO models minimize potential ETO exposure during door opening and load transfer to the aerator. The residual ethylene oxide is then gradually released into the environment over an extended period of time. Griffith while working for Griffith Laboratories devised a process known as the Ethylene Oxide.
Does not apply for EO-sterilized devices that have no patient contact such as in vitro diagnostic devices. The main side effect of using EO as a sterilization agent is that it can leave a residue on the devices being processed. Most modern ETO sterilizers combine sterilization and aeration in the same chamber as a continuous process.
Ethylene oxide sterilization residuals. Ethylene oxide sterilization is an important sterilization method that manufacturers widely use to keep medical devices safe. Materials tend to retain significant amounts of residual ethylene oxide subsequent to a fumigation treatment.
The table below lists allowable limits for terminally sterilized products by Ethylene Oxide. Residual levels of EO and its derivatives ethylene chlorohydrin ECH and ethylene glycol EG may be hazardous to the patients. Because ethylene oxide EO gas is toxic to humans restrictions have been imposed on its use for sterilization specifying allowable levels of residual EO remaining in sterilized apparatus and materials.
Therefore it must be removed by the aeration process. Like 24 hours 48 hours 72 hours etc and filling the GC vials and capping the vials with aluminum seal. Lloyd Augustus Hall a food scientist and a Northwestern University classmate of Carroll L.
Ethylene oxide EO is the sterilization technique generally used for polymer-based medical devices as it can be performed at low-temperatures which leads to minimal changes in molecular weight. EO Sterilant Residual Tests Ethylene Oxide EO Residuals Analysis is used in the identification and quantification of ethylene oxide ethylene chlorohydrin and ethylene glycol by gas chromatography. Mechanical aeration for 8 to 12 hours at 50 to 60C allows desorption of the toxic ETO residual contained in exposed absorbent materials.

China 20 Cbm Ethylene Oxide Sterilizer For Medical Glove China Medical Sterilizer Eo Sterilizer
Ethylene Oxide Sterilizer Buy Biobase

Ethylene Oxide Sterilizer Ethylene Oxide Sterilization Equipment

Ethylene Oxide Sterilizers Telstar
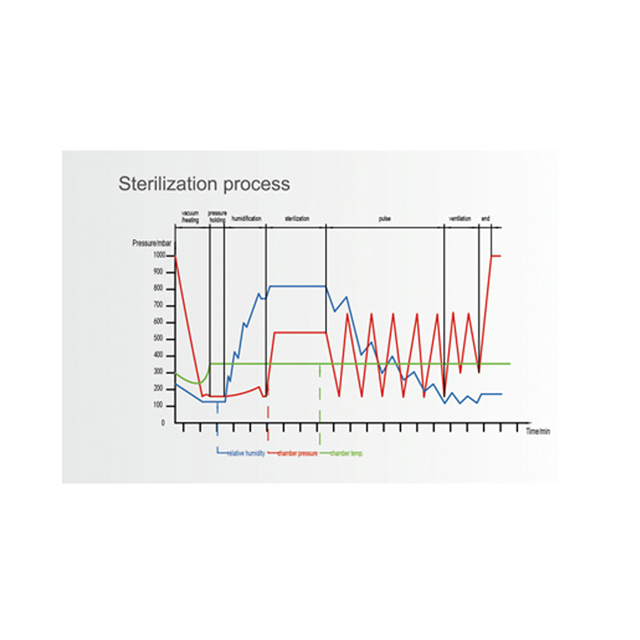
Ethylene Oxide Sterilization Validation Pacific Bio Labs Inc

Pdf Residual Ethylene Oxide In Medical Devices Effects And Estimation Methods An Overview
Ethylene Oxide Sterilization Lexamed

Ethylene Oxide Sterilizers Telstar

China Ethylene Oxide Sterilizer Eto Sterilization Machine Masks Disinfection China Sterilizer Uv Sterilizer
Https Echa Europa Eu Documents 10162 4e5b7970 8517 02ef C757 8f62097fbc10
China Customized Ethylene Oxide Sterilization Equipment Suppliers Manufacturers Factory Bocon

China 1000l Eto Ethylene Oxide Gas Sterilizer Thr 1000b China Ethylene Oxide Gas Sterlizer Eo Gas Sterilizer

Ethylene Oxide Sterilization Equipments And Consumables Xterie
Ethylene Oxide Training On The Hazards Of Ethylene

Pdf Residual Ethylene Oxide In Medical Devices And Device Material

Ethylene Oxide In Sesame Seeds Merieux Nutrisciences Europe
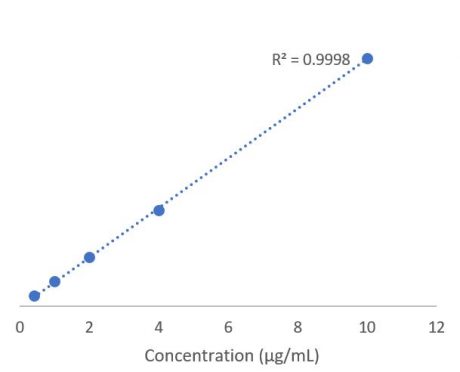
Detection Of Ethylene Oxide Eo Residue In Medical Protective Products

Ethylene Oxide Training On The Hazards Of Ethylene

Ethylene Oxide Sterilization Validation Pacific Bio Labs Inc
0 Response to "Ethylene Oxide Sterilization Residuals"
Posting Komentar